Genetic alterations in somatic and germ cells are associated with serious health effects, which in principle may occur even at low exposure levels.
Mutations in somatic cells may cause cancer if occuring in proto-oncogenes, tumour suppressor genes and/or DNA damage response genes, and are responsible for a variety of genetic diseases.
Accumulation of DNA damage in somatic cells has also been proposed to play a role in degenerative conditions such as accelerated aging, immune dysfunction, cardiovascular and neurodegenerative diseases.
Mutations in germ cells can lead to spontaneous abortions, infertility or heritable damage to the offspring and possibly to the subsequent generations.
Regulatory context
The assessment of the genotoxicity represents an important component of the safety assessment of substances, relevant in the context of EU and international regulations aiming at the protection of human health and the environment.
The results of the genotoxicity tests form the scientific basis for risk assessment and are used for classification and labelling (C&L) of chemical substances in the EU (the Dangerous Substances Directive 67/548/EEC and Regulation (EC) No. 1272/2008 on the classification, labelling and packaging (CLP) of substances and mixtures), and across the world (UN Globally Harmonised System (GHS)).
Several genotoxicity OECD in vitro and in vivo Test Guidelines are already in place which have recently been revised are (OECD TG website).
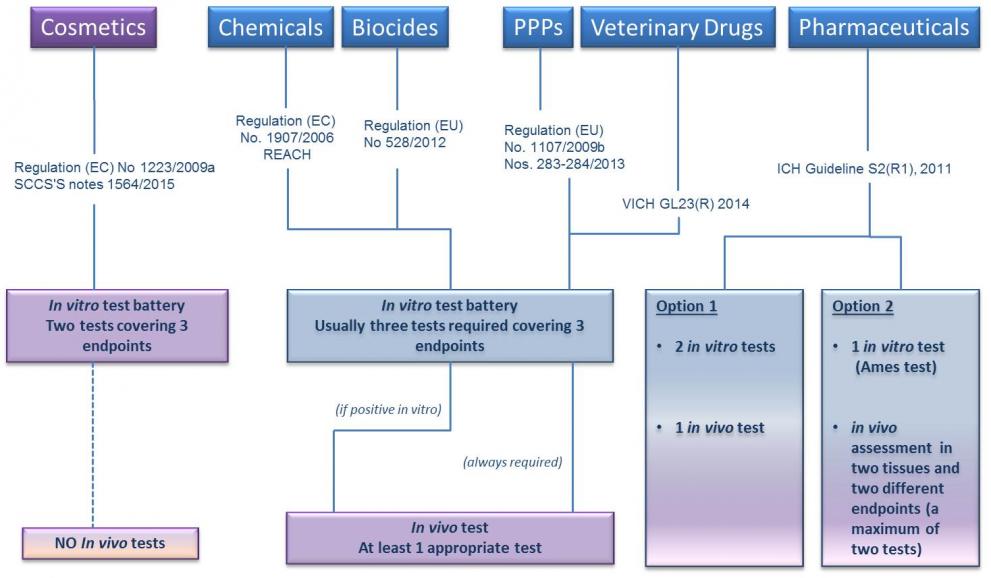
The assessment of genotoxic hazard to humans usually requires that the substance is tested first in the in vitro testing battery.
According to some legislations and guidance documents (e.g. REACH, CLP, Cosmetics Regulation), if a substance is negative in the in vitro battery it is classified as non genotoxic, while a positive outcome in one or more of the in vitro tests requires confirmation by appropriate follow up in vivo testing. Other regulatory requirements (e.g. ICH for pharmaceuticals, Plant Protection Products Regulation) foresee that the in vitro testing battery is always followed up by in vivo testing.
This clearly implies that, although several in vitro tests for genotoxicity assessment are available at different stages of development and acceptance, they cannot be considered to fully replace animal tests needed to evaluate the safety of substances (see Adler et al., 2011).
Development and optimisation of alternative methods and approaches
Main gaps identified: high rate of misleading/false genotoxic positives
Existing in vitro methods, whilst having a high sensitivity (and thus low false negative rate), have a relatively low specificity and thus high rate of false (“misleading”) positive results, which typically has led to unnecessary follow-up testing in vivo for the confirmation of these results.
To address this issue, the recommendations of an ECVAM workshop (Kirkland et al., 2007) have contributed to several international initiatives.
EURL ECVAM strategy to avoid and reduce animal use in genotoxicity testing
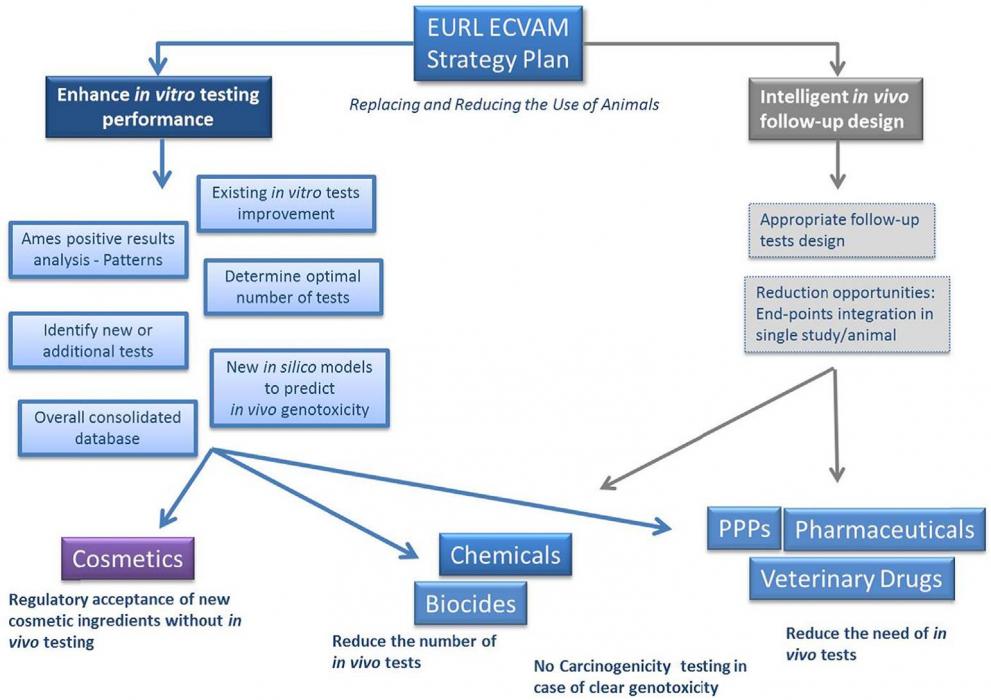
Based on an analysis of regulatory requirements for this endpoint within different pieces of EU legislation, EURL ECVAM has proposed a pragmatic approach to improve the traditional genotoxicity testing paradigm that offers solutions in both the short- and medium-term and that draws on the considerable experience of 40 years of regulatory toxicology testing in this area (EURL ECVAM strategy to avoid and reduce animal use in genotoxicity testing, released in December 2013).
In its strategy, EURL ECVAM has considered that efforts should be directed towards the overall improvement of the current testing strategy for better hazard and risk assessment approaches, which either avoids or minimises the use of animals, whilst satisfying regulatory information requirements, irrespective of regulatory context. Several opportunities for the improvement of genotoxicity testing have been identified which aim to:
- enhance the performance of the in vitro testing battery so that fewer in vivo follow-up tests are necessary and
- guide more intelligent in vivo follow-up testing to reduce unnecessary use of animals.
For a review of the achievements and implementation of the EURL ECVAM strategy plan read Corvi and Madia, 2017.
Are there categories of positive results from the Ames test that are irrelevant or signify low risk?
Differently from in vitro mammalian cell tests, no systematic analysis on Ames test accuracy was conducted until recently, despite the Ames test being the primary test for genotoxicity screening and the most used test within the in vitro battery.
Therefore, a workshop was organized by EURL ECVAM to investigate whether the in vitro mammalian cell genotoxicity test results could complement and mitigate the implications of a positive Ames test response for the prediction of in vivo genotoxicity and carcinogenicity, and if patterns of results could be identified (see workshop report Kirkland et al., 2014a).
The workshop recommended to construct a Genotoxicity & Carcinogenicity Consolidated database (DB) which was consequently launched at the end of 2014. The data collected were analysed to investigate whether negative results in mammalian cell tests were associated with absence of carcinogenic or in vivo genotoxic activity despite a positive Ames test (Kirkland et al., 2014b).
Recommended list of chemicals to assess the performance of new or improved genotoxicity tests
Reference chemical selection is a key step in the development, optimisation and validation of alternative test methods. A revised reference list of genotoxic and non-genotoxic chemicals recommended for assessing the performance of new or improved in vitro genotoxicity test methods has been developed to fit the following different sets of characteristics (Kirkland et al., 2016):
Group 1: Chemicals that should be detected as positive in in vitro mammalian cell genotoxicity tests. Chemicals in this group are all in vivo genotoxins at one or more endpoints, either due to DNA-reactive or non DNA-reactive mechanisms. Many are known carcinogens with a mutagenic mode of action, but a sub-class of probable aneugens has been introduced.
Group 2: Chemicals that should give negative results in in vitro mammalian cell genotoxicity tests. Chemicals in this group are usually negative in vivo and non-DNA-reactive. They are either non-carcinogenic or rodent carcinogens with a non-mutagenic mode of action.
Group 3: Chemicals that should give negative results in in vitro mammalian cell genotoxicity tests, but have been reported to induce gene mutations in mouse lymphoma cells, chromosomal aberrations or micronuclei, often at high concentrations or at high levels of cytotoxicity. Chemicals in this group are generally negative in vivo and negative in the Ames test. They are either non-carcinogenic or rodent carcinogens with an accepted non-mutagenic mode of action.
Reduction
Since substantial in vivo testing is still required by chemical authorities for confirmation of the genotoxic prediction in vitro, it is crucial to address issues related to the reduction and refinement of genotoxicity tests. Recommendations on opportunities to reduce the number of animals in genotoxicity tests are published by Pfuhler and colleagues in an ECVAM workshop report (Pfuhler et al., 2009). Genotoxicity OECD Test Guidelines have recently been revised to also include some animal welfare considerations (OECD TG website).
EURL ECVAM validated test methods
Alongside improvement of the accuracy of existing in vitro methods, and recent validation efforts, several activities are currently in place with the aim of exploring new areas of test development (Corvi and Madia, 2017).
Genotoxicity Assays in 3D
ECVAM has been a partner in a project led by Cosmetics Europe, which aimed to establish and validate new methods for genotoxicity testing in reconstructed human 3D skin models (micronucleus test and comet assay) (Aardema et al., 2010; Pfuhler et al., 2011; Reus et al., 2013).
Comet Assay in vivo
ECVAM has been involved in the Validation Management Team for the validation of the comet assay in vivo, which was coordinated by the Japanese Centre for the Validation of Alternative Methods (JaCVAM) (Uno et al., 2015). The validation of the in vivo assayhas now been finalised and the respective OECD TG 489 has been adopted.
Micronucleus Test (MNT) in vitro
EURL ECVAM has successfully validated the Micronucleus Test (MNT) in vitro, based on a retrospective evaluation of existing data (Corvi et al., 2008). The ECVAM Scientific Advisory Committee (ESAC) endorsed the conclusion that the MNT in vitro is a scientifically valid alternative to the Chromosome Aberration Test in vitro for genotoxicity testing. The in vitro MNT has gained widespread international regulatory acceptance (OECD Test Guideline 487), and is now recommended in several authoritative documents to be used in a core two test battery together with the Ames test (Kirkland et al., 201; COM 2011; EFSA 2011; SSCS/1564/15).
Read more about the Micronucleus Test (MNT) in vitro on TSAR
Reduction
Since substantial in vivo testing is still required by chemical authorities for confirmation of the genotoxic prediction in vitro, it is crucial to address issues related to the reduction and refinement of genotoxicity tests. Recommendations on opportunities to reduce the number of animals in genotoxicity tests are published by Pfuhler and colleagues in an ECVAM workshop report (Pfuhler et al., 2009).
Genotoxicity OECD Test Guidelines have recently been revised to also include some animal welfare considerations (OECD TG website).
Test methods under validation by EURL ECVAM
There are currently no methods undergoing validation in this area.
Scientific tools and databases
The EURL ECVAM Genotoxicity and Carcinogenicity Consolidated Database is a structured master database that compiles available genotoxicity and carcinogenicity data for Ames positive chemicals originating from different sources.
By using a harmonised format to capture the information, this database represents a powerful resource for data analysis that can be used to guide a thorough evaluation of genotoxicity and carcinogenicity.
The database is a living project with possibilities of continuous update as new genotoxicity and carcinogenicity data are made available. The database is currently being extended to include additional curated data from more than 200 new chemicals with Ames negative results.
