The use of genetically modified organisms (GMOs) as food/feed needs to be authorised in Europe. Food/feed products containing more than 0.9% of GMOs have to be labelled (Regulation (EC) No 1829/2003) This labelling threshold applies for adventiously added GMOs, while GMOs added on purpose always need to be labelled. GMOs for which an authorisation procedure is pending or for which the authorisation has expired are allowed in European feed products up to a level of 0.1% (Regulation (EC) No 619/2011)
Quantification of GMOs is required for the implementation of both regulations; consequently they explicitly demand the availability of validated methods and of reference materials. The JRC develops and produces the required reference materials, and hosts the European Reference Laboratory for GM Food and Feed (EU-RL GMFF) where the method validation is carried out. Validated methods and reference materials are important for the standardisation and ensure that the measurements results of laboratories quantifying GMOs are comparable.
GMO reference materials
For the implementation of the European legislation that regulates the authorisation and the labelling of genetically modified organisms (GMOs), the JRC develops, produces and distributes Certified Reference Materials (CRMs). These CRMs are used for the calibration or quality control of GMO quantification measurements, typically carried out by quantitative real-time polymerase chain reaction (qPCR). Numerous sets of reference materials for different GM events in maize, soybean, potato, sugar beet, and cotton are offered to testing and control laboratories world-wide. Research activities are on-going to develop further GMO CRMs, including CRMs for new plant species.
Available GMO Reference Materials
The majority of Certified Reference Materials (CRMs) intended for the quantification of genetically modified organisms (GMOs) are powders produced from seeds or vegetables. These so-called matrix materials are mixtures of non-GMO materials with GMO materials, which have gravimetrically been certified for their mass fraction (expressed in g/kg) of a specific GMO event. The available concentrations differ for the individual GMO event (and the set of CRM) and range from nominal 0 g/kg to 1000 g/kg.
All reference materials available at JRC can be found in the on-line reference materials catalogue.
Development of GMO reference materials
The development of Certified Reference Materials (CRMs) for GMO analysis requires research. Investigations have resulted in major improvements, for instance the possibility to produce powders with a homogenous and sufficiently small particle size using cryo-grinding techniques. Another example is the application of a dry-mixing technique, specifically developed to avoid DNA degradation during the production of a powder CRM. On-going research investigates production tools for stable and homogenous GMO CRMs for further plant species.
JRC also offers to the Biotech Industry a service to develop GMO reference materials.
Use of GMO reference materials
The majority of GMO Certified Reference Materials (CRMs) are powder (matrix) materials and are intended to be used for the calibration or quality control for GMO quantification measurements (CRM code ERM-BF). A few GMO CRMs which exist are limited to the calibration of GMO measurements (CRM code ERM-AD).
Reference materials contribute to the standardisation of measurements and ensures that measurement results are comparable. GMO CRMs are commonly used for the calibration or quality control of GMO quantification measurements based on quantitative real-time Polymerase Chain Reaction (qPCR). Additionally they are well suited for the validation and verification of GMO quantification methods. Their use is recommended in ISO/IEC 17025, while official GMO control laboratories are required to be accredited according to ISO/IEC 17025 (Regulation (EC) No 882/2004).
The JRC has issued a number of application notes dedicated to the proper use of Reference Materials. The application notes of interest for GMO testing laboratories are:
- ERM application note1: Comparison of a measurement result with the certified value
- ERM application note 4: Use of certified reference materials for the quantification of GMO in food and feed.
Guidance on the estimation of measurement uncertainty (MU) in the area of GMO quantification is provided in the guidance document on measurement uncertainty for GMO testing laboratories (3rd edition).
Who does what on GMOs in the JRC?
- JRC’s Development, production and distribution of certified reference materials (CRMs) for analysis of genetically modified organisms (GMOs);
- JRC’s European Reference Laboratory for GM Food and Feed (EU-RL GMFF), including the validation of GMO quantification methods;
Directorate General for Health and Consumers takes care of the EU policy on Genetically Modified Organisms.
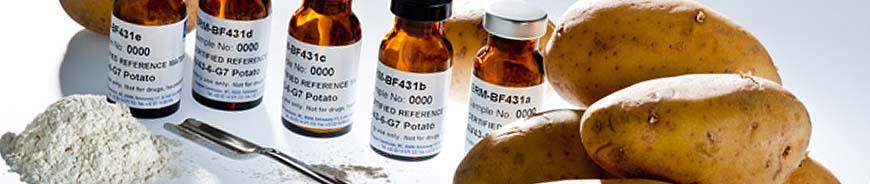
Reference materials for GMO analysis
