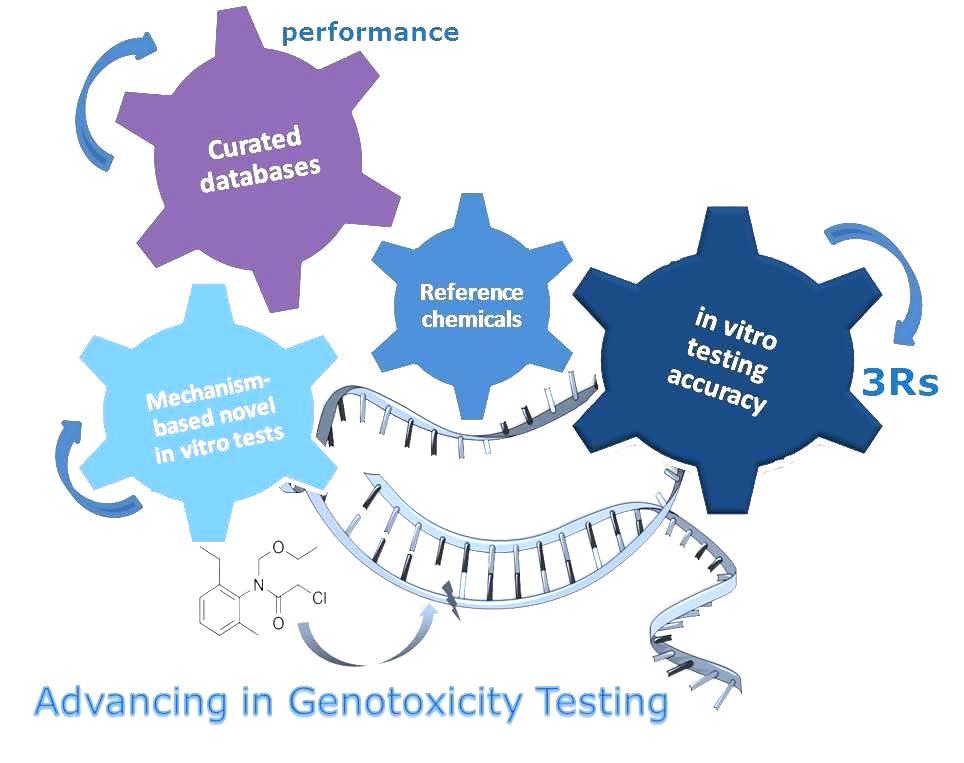
JRC scientists reviewed the recent activities and achievements in the area of genotoxicity testing with the conclusion that the availability of improved tests and strategies can significantly contribute to the reduction and potential replacement of animals used for regulatory testing.
The evaluation of genotoxicity is an essential component of the safety assessment of all types of substances (industrial chemicals, pharmaceuticals, pesticides, food additives, cosmetics ingredients, etc.) for the protection of human and animal health. In general, the assessment of genotoxic hazards to humans begins with non-animal (in vitro) tests followed in some cases by animal testing.
A variety of well-established in vitro assays are available at different stages of development and regulatory acceptance. However, they are not considered at present sufficient to fully replace animal tests currently used to evaluate the safety of substances for regulatory purposes.
A strategy to reduce and avoid animal use in genotoxicity testing had previously been described by the JRC-hosted EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), based on the regulatory needs across different EU legislations, state of the science, and recent efforts undertaken by various organisations.
Moreover, considerable activities have been carried out in the last decade worldwide with the aim of optimising strategies for genotoxicity testing. These recent achievements together with the implementation of the EURL ECVAM strategy plan are described in this review.
They include the improvement of existing tests, the development of novel tests, as well as the establishment and exploration of approaches to optimise in vitro testing accuracy (Kirkland et al. 2014a, Kirkland et al. 2014b). Furthermore, useful tools, such as databases or reference chemical lists, have been developed by the JRC in collaboration with other scientists to support advances in this field. Some of these activities have also led to the revision of OECD Test Guidelines and regulatory guidance.
Read more in: Corvi F and Madia R: "In vitro genotoxicity testing–Can the performance be enhanced?" Food Chem. Toxicol. 106 (2017), 600-608. doi: 10.1016/j.fct.2016.08.024
Related Content
In vitro genotoxicity testing–Can the performance be enhanced?
Details
- Publication date
- 28 August 2017
