The JRC has put together a COVID-19 In Vitro Diagnostic Devices and Test Methods Database, compiling information on COVID-19 test devices and making it publicly available.
Along with the vaccination drive, large-scale testing has become a key tool in the fight against the pandemic.
Health professionals and users alike, however, face a dizzying array of test devices to choose from, after thousands of them had come onto the market.
Recognising the problem, the European Commission called, in April 2020, for a “centralised overview of available information on test performance and act as a single point of contact for management of this information”.
The JRC created this overview, from which it developed the COVID-19 In Vitro Diagnostic Devices and Test Methods Database, a one-stop shop collecting information on COVID-19 test kits and methods.
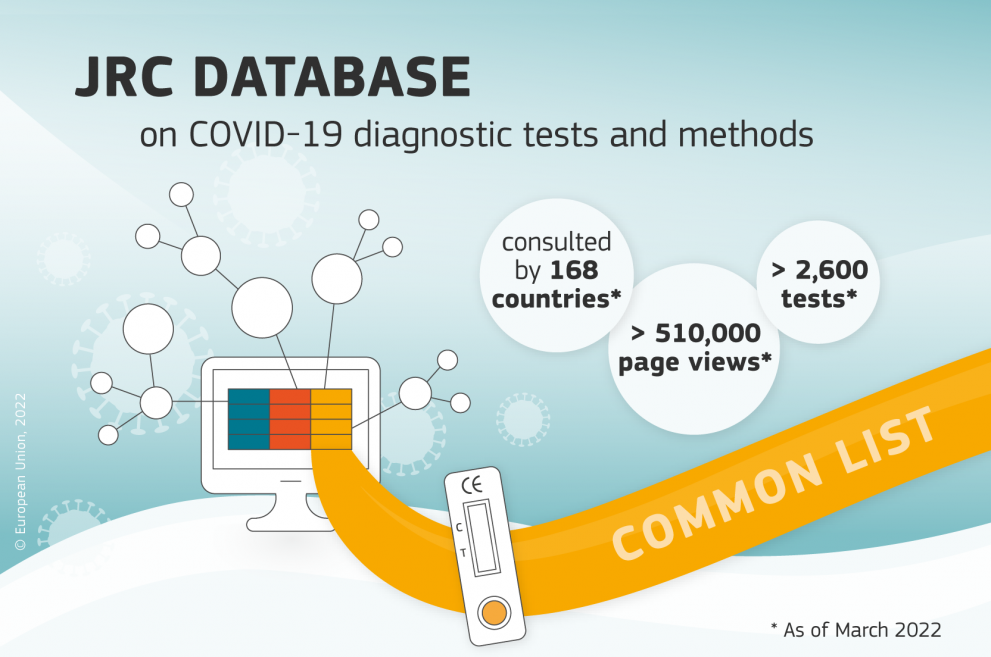
In just a short time, the database has become one of the most complete inventories of COVID-19 test methods and devices worldwide, covering over 2600 in total.
Data in the inventory, which are provided by the manufacturers, include performance details of individual devices and methods, be they polymerase chain reaction (PCR) methods, rapid antigen tests (RAT) or antibody tests.
Such performance details describe the precision (how close repeated measurements are to each other), accuracy (how close a measurement is to the true value), clinical sensitivity (ability to identify patients with the disease), and specificity (ability to spot those who do not have the disease) of the tests.
The EU Digital COVID-19 Certificate – streamlining travelling
The database has been contributing to the development and maintenance of the Health Security Committee’s EU common list of COVID-19 rapid antigen tests that are admissible for the purposes of issuing EU Digital COVID-19 certificates.
The certificate streamlines travelling across the EU by making sure that a single document can be used to prove one’s much-reduced likelihood of carrying the SARS-CoV-2 virus, as the rapid antigen tests included in the EU common list are mutually recognised by all member states.
This EU common list of rapid antigen tests is regularly updated by the Health Security Committee’s technical working group on COVID-19 diagnostic devices, based on proposals received from manufacturers and Member States for new tests to be included. These proposals are submitted through the JRC database and are assessed by the technical working group against a set of pre-defined criteria.
In addition to its significance in the EU, the database also proved to be practical beyond European borders: 168 countries have made use of the database so far, with over 510,000 page views.
Detecting variants of concern
The question whether existing tests are still able to detect mutation of the virus has been accompanying the fight on the pandemic since even minor mutations can affect detectability.
For this reason, the JRC supports laboratories that use COVID-19 detection methods and devices. With the help of advanced bioinformatics tools, JRC experts, on the basis of fully sequenced genomes of COVID-19, simulate if the assays perform well, or if mutations have compromised detectability, making sure that the moving target of COVID-19 identification stays in focus. Results of these in silico analyses are made available through a dedicated dashboard on the database website.
In December 2021, the JRC pioneered an Omicron-specific detection method, enabling laboratories to carry out standard PCR tests to identify the Omicron variant quickly and without the need for time-consuming sequencing.
Earlier, the JRC was instrumental in building the European Sewage Sentinel System for SARS-CoV-2, an EU-wide effort to gauge the presence of the virus in the population by sampling wastewater.
Related content
Sources
Details
- Publication date
- 11 March 2022
- Author
- Joint Research Centre
