The outcome of a workshop reviewing progress in carcinogenicity assessment of chemicals has been published in the latest issue of Toxicology in Vitro Special Issue. The workshop was organised by the JRC's EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and the European Society of Toxicology In Vitro (ESTIV) and identified opportunities for exploiting recent advances in test methods and assessment approaches to move away from the two-year cancer bioassay in rodents.
The workshop took place on the occasion of the 19th ESTIV Congress in Juan Les Pins in October 2016 and opened with the presentation of a recent analysis conducted by JRC scientists of carcinogenicity testing for regulatory purposes in the EU (Madia F., Worth A., and Corvi R. (2016). Analysis of carcinogenicity testing for regulatory purposes in the European Union. EUR 27765. Publications Office of the European Union, Luxembourg.).
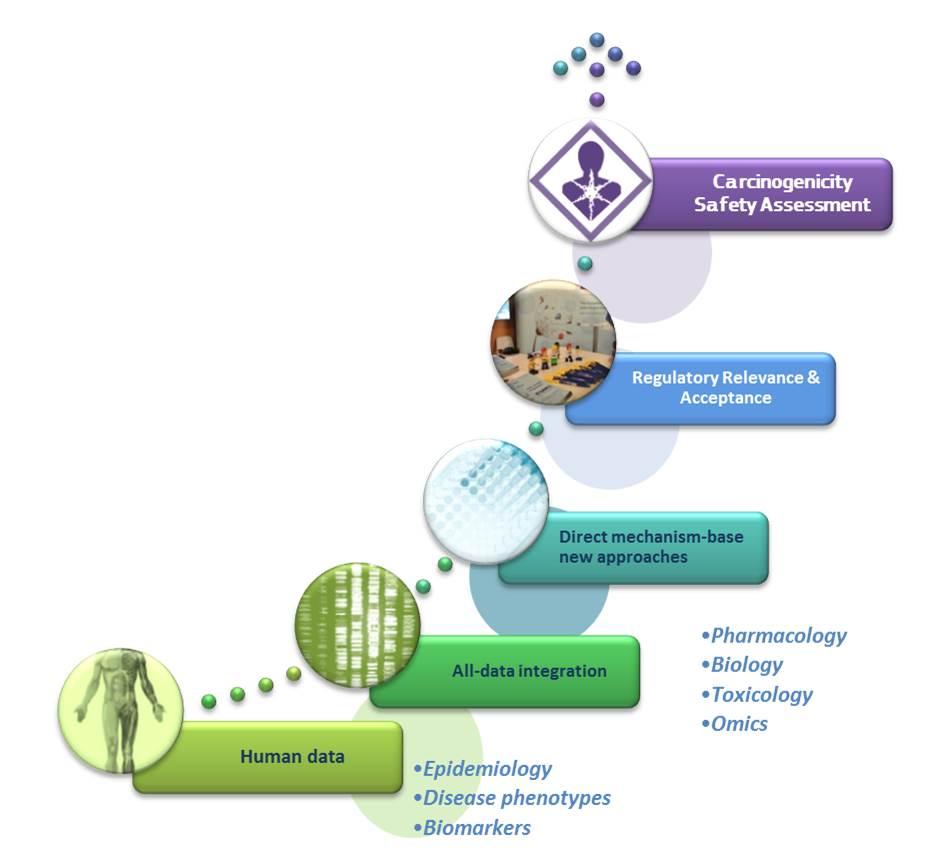
This was followed by presentations of a number of international initiatives including the International Conference on Harmonization (ICH) approach for pharmaceuticals to waive the cancer study and the recent revised methodology for the systematic classification of carcinogens by the International Agency for Research on Cancer (IARC). Attention was also given to how integrating different -omics and high throughput technologies together with disease knowledge into more traditional testing.
Despite a seemingly diverse range of strategic developments across various initiatives and sectors, a number of common critical elements emerged such as the clear need to incorporate mechanistic reasoning into carcinogenicity hazard assessment for all types of substances, and the push towards the integration of all available information including data from epidemiology, traditional and alternative toxicology tests, and from novel data streams (e.g., 'omics, high through technologies).
Such cross-sectorial knowledge sharing and harmonization will build confidence in new approach methods and ultimately lead to more effective and efficient safety assessment.
Read more in: Corvi R. et al.: "Moving forward in carcinogenicity assessment: Report of an EURL ECVAM/ESTIV workshop". Toxicology in Vitro 45 (2017) 278-286. doi:10.1016/j.tiv.2017.09.010
In the same issue, JRC scientists, in close collaboration with the Universities of Milan and Florence in Italy, propose an improved statistical model to investigate the carcinogenic potential of chemicals using the cell transformation assay (CTA) and digital imaging analysis.
Read more in: Callegaro G. et al.: "A comprehensive statistical classifier of foci in the Cell Transformation Assay for carcinogenicity testing". Toxicology in Vitro 45 (2017):351-358. doi: 10.1016/j.tiv.2017.04.030
Read more here for an overview of the Toxicology in Vitro Special Issue .
Related Content
Moving forward in carcinogenicity assessment: Report of an EURL ECVAM/ESTIV workshop
Details
- Publication date
- 17 January 2018
