Scientists from the JRC and US Environmental Protection Agency examined how and to what extent mathematical models of biological organisms are used in toxicology, and identified a number of challenges that need to be overcome for wider use and regulatory acceptance of these non-animal approaches.
The survey aimed to understand how mathematical models of biological organisms are being used to support chemical risk assessment in a variety of sectors such as pesticides, industrial chemicals and consumer products. These models help to refine an assessment while reducing the reliance on animal testing. However, a number of limitations and barriers were identified that need to be addressed to increase the uptake of these models.
The survey focussed on the use of physiologically based kinetic (PBK) models which provide a mathematical representation of how chemicals are distributed in the body following exposure. This information - what the body does to a chemical – is used along with additional information, e.g. from in vitro toxicity experiments, to determine the potential health risks resulting from realistic exposure conditions.
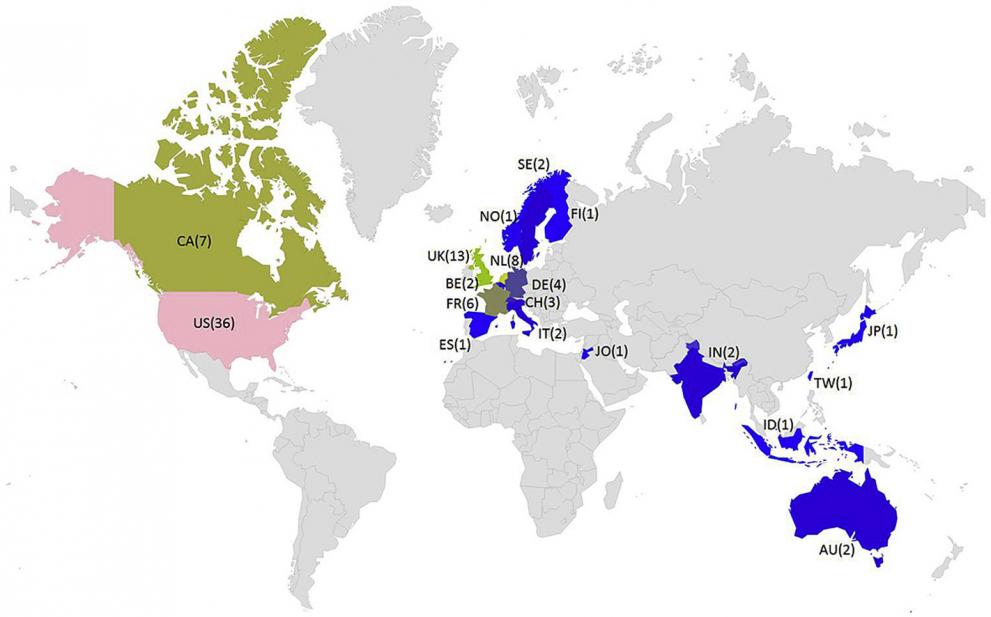
The results of the survey indicate that PBK models are used widely in academia, industry, and government. Moreover, significant scientific and technical advances have been made in their development and application. There remains some reluctance, however, among regulatory agencies to apply these models to their full potential. One reason for this is the lack of appropriate guidance. The findings were based on the responses of 93 individuals (modelling experts and end-users) from 19 countries. The individual results are publicly accessible at: http://bolegweb.geof.unizg.hr/questionaire/pbk
Moreover, with a view to increasing the acceptance and use of PBK models by regulatory authorities, the JRC and US EPA will co-lead a new project within the Organisation for Economic Cooperation and Development (OECD) in order to harmonise the characterisation, validation and reporting of PBK models.
This project will draw on best practises in PBK modelling, such as those identified by the survey respondents.
Read more in: A Paini et al (2017), Investigating the state of physiologically based kinetic modelling practices and challenges associated with gaining regulatory acceptance of model applications, Regulatory Toxicology and Pharmacology 90, 104-115, doi: 10.1016/j.yrtph.2017.08.019
Related Content
Details
- Publication date
- 13 November 2017
